Stephen Beebe

Research Professor
PHONE: 757-683-2405
EMAIL: sbeebe@odu.edu
ADDRESS: IRP 2, 4211 Monarch Way, Norfolk, VA, 23508
Education
- Postdoctoral fellow and research associate: Vanderbilt University in the Howard Hughes Medical Institute 1982-1987
- Ph.D.: Medical College of Ohio Medical Sciences (Pharmacology) 1982; Now-Toledo University School of Medicine
- B.S.: Ohio University, 1970 B.S. Zoology
Lab Members
Kamal Asadipour, Ph.D. Student
Research Description
Laboratory for Molecular Cell Signaling in Cancer Therapeutics and Bioelectrics has two general strategies, in vitro and in vivo (or ex vivo), that determine how nanosecond pulsed electric fields (nsPEFs) or Nanopulse Stimulation (NPS) induce immunogenic cell death (ICD) in tumors. ICD causes tumor death and in situ vaccinates animals after treatment in two orthotopic cancer models, N1-S1 rat liver and 4T1-luc mouse breast cancer. Another strategy includes investigating why mouse B16f10 melanoma tumors are not as effectively eliminated by NPS, why mice are not as readily in situ vaccinated, and why immune responses are not as effective against melanoma as the liver and breast models after nsPEF treatment.
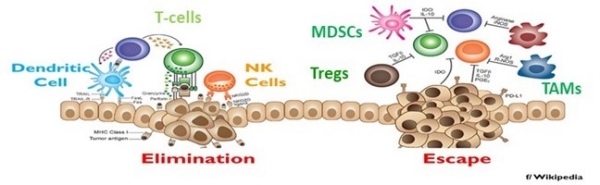
The image shows options for tumor elimination or growth through escape from the host's immune system. NPS as immunotherapy requires (1) tumor elimination; (2) induction of innate and adaptive immune responses by activation of natural killer (NK) cells, dendritic cells, and T-cells; and (3) prevention of immune escape by relieving immunosuppression in the tumor microenvironment (TME) by decreasing numbers of T-regulatory cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages, which promote immune suppression.
Published and ongoing studies indicate that the liver [Lassiter et al., 2018] and breast cancer models [Guo et al., 2018; Beebe et al., 2018] successfully induce in situ vaccination because they effectively employ the three immunotherapeutic processes indicated above. They eliminate tumors, activate T-cell, NK cells, and dendritic cell responses against the tumor cells, and relieve the immunosuppressive TME. In the B16f10 melanoma model, NPS activates T-cell responses against the B16f10 tumor cells, but does not effectively relieve immunosuppression in the TME after eliminating B16f10 tumors. Immunosuppressive cells return to the TME on days 3 and 7 after an initial decrease on day one post-treatment.
Two new strategies beginning in 2022 include (1) preventing the return of the immunosuppressive cells to the TME in the B16f10 model by preventing the recruitment of circulating monocytes that become corrupted and induce immunosuppression to the remaining TME. Second, the continuation of TME immunosuppression may suggest that NPS may need additional support to prevent tumor regrowth. So we are beginning studies that inject carbon nanotubes (CNT) into the TME to enhance the electric fields there and lower the need for high voltages, which will be helpful for NPS application in the clinic setting. We will also functionalize these CNT with antibodies to PDL-1, a checkpoint inhibitor that promotes immunosuppression by tumor cells.
In vitro studies focus on the mitochondria as an NPS target for ICD induction. A working hypothesis suggest that NPS induces mitochondrial Ca2+ influx (overload) because of nanoporation of the plasma membrane and a mitochondrial increase in reactive oxygen species (mROS) that opens the mitochondrial permeability transition pore (mPTP). We propose that the rise in mROS is caused by a nsPEF-induced attenuation of electron (and proton) transport in the mitochondrial electron transport chain (ETC)at complex I as determined by a decrease in O2 utilization, which in the presence of Ca2+ activation of NADH production through the Krebs cycle {L Potter, PhD thesis], causes reverse electron transport and increased ROS production at complex I. Another possibility is that NPS activates and opens the mPTP by another mechanism (conformational change), and this effect causes the increase in ROS and attenuation of electron/proton transport in the ETC complex I. We are also investigating nsPEFs effects to modify mitochondrial morphology by fission, which modulates metabolism. Metabolic studies also indicate that NPS in vivo-activated CD4 exhibit metabolism of activated immune cell phenotype [B Ruedlinger, PhD thesis].
NsPEFs also modulates electron transport not only in mitochondria ETC but also in the plasma membrane redox system (PMRS). Regulation of electron transport presents a new paradign for nsPEF effects on biologival cells. The trans plasma membrane electron transport (tPMET) in the PMRS is increased at low pulse conditions and decreased at high pulse conditions. This biphasic regulation is typical of hormesis, where a biphasic dose-response to an environmental stimulus characterized by a low dose stimulation or beneficial effect and a high dose inhibitory or toxic effect. Mechanistically the beneficial effects of nsPEFs on tPMET include maintenance of redox homeostasis with relief from external oxidant stress and internal reductive stresses and the facilitation of metabolic flux through glycolysis as NAD(P)+ is regenerated. This is especially significant in malignant cells. A nsPEF-induced decrease in tPMET in cancer cells is closely correlated with loss of cell viability because of the importance of tPMET for normal cell funcrions. This correlation is less striking in non-cancerous cells that mostly derive energy from mitochondrial oxidative phiosphorlation.
Most Relevant Publications
- Schoenbach KH, Beebe SJ, Buescher ES. Intracellular Effect of Ultrashort Electrical Pulses. Bioelectromagnetics Sept 2001;22(6):440-448. [725 Citations]
- Beebe SJ, Fox PM, Rec LJ, Willis LK, Schoenbach KH. Nanosecond, high intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003;17:1493-1495. [509 Citations].
- Beebe SJ, Chen YJ, Sain NM, Schoenbach KH, Xiao S. Transient features in nanosecond pulsed electric fields differentially modulate mitochondria and viability. PLoS One. 2012;7(12):e51349 [77 Citations]
- S Guo, Y Jing, NI Burcus, BP Lassiter, R Tanaz, R Heller, SJ Beebe. Nano‐pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. International journal of cancer. 2018; 142 (3), 629-640. [53 Citations].
- Lassiter BP, Guo S, Beebe SJ. Nano-Pulse Stimulation Ablates Orthotopic Rat Hepatocellular Carcinoma and Induces Innate and Adaptive Memory Immune Mechanisms that Prevent Recurrence. Cancers. 2018;10. pii: E69. [22 Citations]
- Stephen J. Beebe, Ravi Joshi, Karl H. Schoenbach, Shu Xiao. Ultrashort Electric Pulse Effects in Biology and Medicine. Springer, Singapore 2021 - Series in BioEngineering. eBook ISBN 978-981-10-5113-5. Hardcover ISBN 978-981-10-5112-8; Series ISSN 2196-8861
Awards and Honors
- Pre-doctoral Fellowship Award; Medical College of Ohio -1977-1981
- Howard Hughes Medical Institute Post-Doctoral Fellowship 1982-1985
- Fulbright Scholar Award, Fulbright Professor, Oslo, Norway 1987-1988
- Marshall Scholar Award, Oslo, Norway 1988
- Norwegian Research Council: Outstanding Senior Visiting Scientist, Bergen, Norway 1997-1999
- Iwao Yasuda Award -excellence in biomedical research in physical regulation in biology and medicine - from the Society for Physical Regulation in Biology and Medicine 2002
- The Martin Black Prize from the Institute of Physics and Engineering in Medicine: SJ Beebe et al. best paper published in Physiological Measurement in 2004 - in Physiol Meas. 2004;25:1077-1093
- The Frank Reidy Award for Outstanding Achievements in Bioelectrics - 2017
- In the top 2% of most-cited scientists in his field in 2020 - Stanford study

